Periodic Table Extended
I have to apologize. The Original Arakish used the 32-column PT. Actually more sensible for how he plotted his SEEs.
Author’s Note: I finally lowered font size to 0.8em for this page width. This is the HTML version of the table Arakish designed on graph paper almost ¾-century ago. I took liberty to replace current AS for elements 100-108. His only went to element-99, Einsteinium.
|
1 H |
2 He |
2 He |
|||||||||||||||||||||||||||||
|
3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||||||||||||||||
|
11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
||||||||||||||||||||||||
|
19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
||||||||||||||
|
37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
||||||||||||||
|
55 Cs |
56 Ba |
57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
|
87 Fr |
88 Ra |
89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og |
|
119 |
120 |
121 |
122 |
123 |
124 |
125 |
126 |
127 |
128 |
129 |
130 |
131 |
132 |
133 |
134 |
135 |
136 |
137 |
138 |
139 |
140 |
141 |
142 |
143 |
144 |
145 |
146 |
147 |
148 |
149 |
150 |
|
151 |
152 |
153 |
154 |
155 |
156 |
157 |
158 |
159 |
160 |
161 |
162 |
163 |
164 |
165 |
166 |
167 |
168 |
169 |
170 |
171 |
172 |
173 |
174 |
175 |
176 |
177 |
178 |
179 |
180 |
181 |
182 |
|
183 |
184 |
185 |
186 |
187 |
188 |
189 |
190 |
191 |
192 |
193 |
194 |
195 |
196 |
197 |
198 |
199 |
200 |
201 |
202 |
203 |
204 |
205 |
206 |
207 |
208 |
209 |
210 |
211 |
212 |
213 |
214 |
|
215 |
216 |
217 |
218 |
219 |
220 |
221 |
222 |
223 |
224 |
225 |
226 |
227 |
228 |
229 |
230 |
231 |
232 |
233 |
234 |
235 |
236 |
237 |
238 |
239 |
240 |
241 |
242 |
243 |
244 |
245 |
246 |
|
247 |
248 |
249 |
250 |
251 |
252 |
253 |
254 |
255 |
256 |
257 |
258 |
259 |
260 |
261 |
262 |
263 |
264 |
265 |
266 |
267 |
268 |
269 |
270 |
271 |
272 |
273 |
274 |
275 |
276 |
277 |
278 |
|
279 |
280 |
281 |
282 |
283 |
284 |
285 |
286 |
287 |
288 |
289 |
290 |
291 |
292 |
293 |
294 |
295 |
296 |
297 |
298 |
299 |
300 |
301 |
302 |
303 |
304 |
305 |
306 |
307 |
308 |
309 |
310 |
|
311 |
312 |
313 |
314 |
315 |
316 |
317 |
318 |
319 |
320 |
321 |
322 |
323 |
324 |
325 |
326 |
327 |
328 |
329 |
330 |
331 |
332 |
333 |
334 |
335 |
336 |
337 |
338 |
339 |
340 |
341 |
342 |
|
343 |
344 |
345 |
346 |
347 |
348 |
349 |
350 |
351 |
352 |
353 |
354 |
355 |
356 |
357 |
358 |
359 |
360 |
361 |
362 |
363 |
364 |
365 |
366 |
367 |
368 |
369 |
370 |
371 |
372 |
373 |
374 |
| 375 | 376 | 377 | 378 | 379 | 380 | 381 | 382 | 383 | 384 | 385 | 386 | 387 | 388 | 389 | 390 | 391 | 392 | 393 | 394 | 395 | 396 | 397 | 398 | 399 | 400 | 401 | 402 | 403 | 404 | 405 | 406 |
| 407 | 408 | 409 | 410 | 411 | 412 | 413 | 414 | 415 | 416 | 417 | 418 | 419 | 420 | 421 | 422 | 423 | 424 | 425 | 426 | 427 | 428 | 429 | 430 | 431 | 432 | 433 | 434 | 435 | 436 | 437 | 438 |
| 439 | 440 | 441 | 442 | 443 | 444 | 445 | 446 | 447 | 448 | 449 | 450 | 451 | 452 | 453 | 454 | 455 | 456 | 457 | 458 | 459 | 460 | 461 | 462 | 463 | 464 | 465 | 466 | 467 | 468 | 469 | 470 |
| 471 | 472 | 473 | 474 | 475 | 476 | 477 | 478 | 479 | 480 | 481 | 482 | 483 | 484 | 485 | 486 | 487 | 488 | 489 | 490 | 491 | 492 | 493 | 494 | 495 | 496 | 497 | 498 | 499 | 500 | 501 | 502 |
| 503 | 504 | 505 | 506 | 507 | 508 | 509 | 510 | 511 | 512 | 513 | 514 | 515 | 516 | 517 | 518 | 519 | 520 | 521 | 522 | 523 | 524 | 525 | 526 | 527 | 528 | 529 | 530 | 531 | 532 | 533 | 534 |
| 535 | 536 | 537 | 538 | 539 | 540 | 541 | 542 | 543 | 544 | 545 | 546 | 547 | 548 | 549 | 550 | 551 | 552 | 553 | 554 | 555 | 556 | 557 | 558 | 559 | 560 | 561 | 562 | 563 | 564 | 565 | 566 |
| 567 | 568 | 569 | 570 | 571 | 572 | 573 | 574 | 575 | 576 | 577 | 578 | 579 | 580 | 581 | 582 | 583 | 584 | 585 | 586 | 587 | 588 | 589 | 590 | 591 | 592 | 593 | 594 | 595 | 596 | 597 | 598 |
| 599 | 600 | 601 | 602 | 603 | 604 | 605 | 606 | 607 | 608 | 609 | 610 | 611 | 612 | 613 | 614 | 615 | 616 | 617 | 618 | 619 | 620 | 621 | 622 | 623 | 624 | 625 | 626 | 627 | 628 | 629 | 630 |
| 631 | 632 | 633 | 634 | 635 | 636 | 637 | 638 | 639 | 640 | 641 | 642 | 643 | 644 | 645 | 646 | 647 | 648 | 649 | 650 | 651 | 652 | 653 | 654 | 655 | 656 | 657 | 658 | 659 | 660 | 661 | 662 |
| 663 | 664 | 665 | 666 | 667 | 668 | 669 | 670 | 671 | 672 | 673 | 674 | 675 | 676 | 677 | 678 | 679 | 680 | 681 | 682 | 683 | 684 | 685 | 686 | 687 | 688 | 689 | 690 | 691 | 692 | 693 | 694 |
| 695 | 696 | 697 | 698 | 699 | 700 | 701 | 702 | 703 | 704 | 705 | 706 | 707 | 708 | 709 | 710 | 711 | 712 | 713 | 714 | 715 | 716 | 717 | 718 | 719 | 720 | 721 | 722 | 723 | 724 | 725 | 726 |
| 727 | 728 | 729 | 730 | 731 | 732 | 733 | 734 | 735 | 736 | 737 | 738 | 739 | 740 | 741 | 742 | 743 | 744 | 745 | 746 | 747 | 748 | 749 | 750 | 751 | 752 | 753 | 754 | 755 | 756 | 757 | 758 |
| 759 | 760 | 761 | 762 | 763 | 764 | 765 | 766 | 767 | 768 | 769 | 770 | 771 | 772 | 773 | 774 | 775 | 776 | 777 | 778 | 779 | 780 | 781 | 782 | 783 | 784 | 785 | 786 | 787 | 789 | 790 | 791 |
| 792 | 793 | 794 | 795 | 796 | 797 | 798 | 799 | 800 | 801 | 802 | 803 | 804 | 805 | 806 | 807 | 808 | 809 | 810 | 811 | 812 | 813 | 814 | 815 | 816 | 817 | 818 | 819 | 820 | 821 | 822 | 823 |
| 824 | 825 | 826 | 827 | 828 | 829 | 830 | 831 | 832 | 833 | 834 | 835 | 836 | 837 | 838 | 839 | 840 | 841 | 842 | 843 | 844 | 845 | 846 | 847 | 848 | 849 | 850 | 851 | 852 | 853 | 854 | 855 |
| 856 | 857 | 858 | 859 | 860 | 861 | 862 | 863 | 864 | 865 | 866 | 867 | 868 | 869 | 870 | 871 | 872 | 873 | 874 | 875 | 876 | 877 | 878 | 879 | 880 | 881 | 882 | 883 | 884 | 885 | 886 | 887 |
| 888 | 889 | 890 | 891 | 892 | 893 | 894 | 895 | 896 | 897 | 898 | 899 | 900 | 901 | 902 | 903 | 904 | 905 | 906 | 907 | 908 | 909 | 910 | 911 | 912 | 913 | 914 | 915 | 916 | 917 | 918 | 919 |
| 920 | 921 | 922 | 923 | 924 | 925 | 926 | 927 | 928 | 929 | 930 | 931 | 932 | 933 | 934 | 935 | 936 | 937 | 938 | 939 | 940 | 941 | 942 | 943 | 944 | 945 | 946 | 947 | 948 | 949 | 950 | 951 |
AUTHOR’S NOTE: REMEMBER, the above extension is only showing the number of protons, the Atomic Number, Z.
If I ain’t mistaken, I think this guy was trying to see if there was any pattern to the “magic numbers” within the 32-col Periodic Table, and his extension of it. However, even to me there appears to be no pattern. Perhaps if the PT were extended into 10,000 and above? But I ain’t gonna do it.
Wild Tangent: Lead is the ultimate cause for the rise in autism in today’s world. We ALL KNOW lead poisoning can cause mental retardation. Autism is just another name for the same thing. T…H…I…N…K… about THAT. Autism is even in the DSM-V. Go look. We poisoned the biosphere with lead for over 100 years during our Industrial Era. Now it bites us humans in the arse. And it will be around for much, much longer. Perhaps, at least, for another 100+ years, up to 1Ka.
Wild Tangent: Want to know how we finally figured out that the dinosaurs were actually endotherms? Warm-blooded? Oxygen.
Addenda: Found this excellent nuclide page – https://www.nndc.bnl.gov/nudat3/Nuclide Map

When you look at the nuclide (isotope) map, you see oxygen has three stable forms: O16, O17, O18. They list them in an easier fashion: 16O, 17O, 18O. F is fluorine, N is nitrogen, C is carbon.
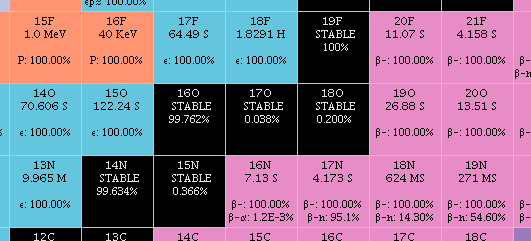
Endothermic animals maintain a ratio of O16:O18. Ectothermic (cold-blooded) animals do NOT maintain such a ratio. Then the O18, is incorporated into the bones. We found the bones of the dinosaur fossils had the same O16:O18 ratio as us humans have today. Thus, the dinosaurs were NOT the slowly plodding cold-blooded reptiles we thought. They were warm-blooded, and savagely fast and quick. Except the herbivores. 7734, a Tyrannosaur could very well have had a short-dash speed of 60kph (37.28mph)! Then, how short is short? A kilometer or two? No hope of out-running it in the open. And as mentioned in the movie Jurassic Park by Robert Muldoon, Velociraptors could hit cheetah speed: 90-130kph (56-81mph). But that would be their Dash speed. Their Run speed could easily be maintained at 40kph easy, which is the human Dash speed. Even Usain Bolt only topped out at about 37½kph.
Some Elemental Densities
forthcoming, eventually| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen | 1 | H | 1 | 0 | 1.008 | 0.00008988 | 14.01 | 20.28 | s- | gas | |
| Helium | 2 | He | 2 | 2 | 4.0026 | 0.0001785 | 4.22 | s- | gas | ||
| Lithium | 3 | Li | 3 | 3 | 6.94 | 0.534 | 453.69 | 1560 | s- | solid | alkali |
| Beryllium | 4 | Be | 4 | 4 | 9.0122 | 1.85 | 1560 | 2742 | s- | solid | alkaline |
| Boron | 5 | B | 5 | 6 | 10.81 | 2.34 | 2349 | 4200 | p- | solid | metalloid |
| Carbon | 6 | C | 6 | 6 | 12.011 | 2.267 | >4000 | 4300 | p- | solid | mineral |
| Nitrogen | 7 | N | 7 | 7 | 14.007 | 0.0012506 | 63.15 | 77.36 | p- | gas | |
| Oxygen | 8 | O | 8 | 8 | 15.999 | 0.001429 | 54.36 | 90.2 | p- | gas | |
| Fluorine | 9 | F | 9 | 10 | 18.998 | 0.001696 | 53.53 | 85.03 | p- | gas | halogen |
| Neon | 10 | Ne | 10 | 10 | 20.18 | 0.0009002 | 24.56 | 27.07 | p- | gas | inert |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Sodium | 11 | Na | 11 | 12 | 22.99 | 0.968 | 370.87 | 1156 | s- | solid | alkali |
| Magnesium | 12 | Mg | 12 | 12 | 24.305 | 1.738 | 923 | 1363 | s- | solid | alkaline |
| Aluminium | 13 | Al | 13 | 14 | 26.982 | 2.7 | 933.47 | 2792 | p- | solid | metal |
| Silicon | 14 | Si | 14 | 14 | 28.085 | 2.329 | 1687 | 3538 | p- | solid | metalloid |
| Phosphorus | 15 | P | 15 | 16 | 30.974 | 1.823 | 317.3 | 550 | p- | solid | mineral |
| Sulfur | 16 | S | 16 | 16 | 32.06 | 2.07 | 388.36 | 717.87 | p- | solid | mineral |
| Chlorine | 17 | Cl | 17 | 18 | 35.45 | 0.0032 | 171.6 | 239.11 | p- | gas | halogen |
| Argon | 18 | Ar | 18 | 22 | 39.95 | 0.001784 | 83.8 | 87.3 | p- | gas | inert |
| Potassium | 19 | K | 19 | 20 | 39.098 | 0.89 | 336.53 | 1032 | s- | solid | alkali |
| Calcium | 20 | Ca | 20 | 20 | 40.078 | 1.55 | 1115 | 1757 | s- | solid | alkaline |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Scandium | 21 | Sc | 21 | 24 | 44.956 | 2.985 | 1814 | 3109 | d- | solid | Rare Earth |
| Titanium | 22 | Ti | 22 | 26 | 47.867 | 4.506 | 1941 | 3560 | d- | solid | metal |
| Vanadium | 23 | V | 23 | 28 | 50.942 | 6.11 | 2183 | 3680 | d- | solid | metal |
| Chromium | 24 | Cr | 24 | 28 | 51.996 | 7.15 | 2180 | 2944 | d- | solid | metal |
| Manganese | 25 | Mn | 25 | 30 | 54.938 | 7.21 | 1519 | 2334 | d- | solid | metal |
| Iron | 26 | Fe | 26 | 30 | 55.845 | 7.874 | 1811 | 3134 | d- | solid | metal |
| Cobalt | 27 | Co | 27 | 32 | 58.933 | 8.9 | 1768 | 3200 | d- | solid | metal |
| Nickel | 28 | Ni | 28 | 31 | 58.693 | 8.908 | 1728 | 3186 | d- | solid | metal |
| Copper | 29 | Cu | 29 | 35 | 63.546 | 8.96 | 1357.77 | 2835 | d- | solid | metal |
| Zinc | 30 | Zn | 30 | 35 | 65.38 | 7.14 | 692.88 | 1180 | d- | solid | metal |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Gallium | 31 | Ga | 31 | 39 | 69.723 | 5.91 | 302.9146 | 2673 | p- | solid | metal |
| Germanium | 32 | Ge | 32 | 41 | 72.63 | 5.323 | 1211.4 | 3106 | p- | solid | metalloid |
| Arsenic | 33 | As | 33 | 42 | 74.922 | 5.727 | 1090 | 887 | p- | solid | metalloid |
| Selenium | 34 | Se | 34 | 45 | 78.971 | 4.81 | 453 | 958 | p- | solid | metal |
| Bromine | 35 | Br | 35 | 45 | 79.904 | 3.1028 | 265.8 | 332 | p- | gas | halogen |
| Krypton | 36 | Kr | 36 | 48 | 83.798 | 0.003749 | 115.79 | 119.93 | p- | gas | inert |
| Rubidium | 37 | Rb | 37 | 48 | 85.468 | 1.532 | 312.46 | 961 | s- | solid | alkali |
| Strontium | 38 | Sr | 38 | 50 | 87.62 | 2.64 | 1050 | 1655 | s- | solid | alkaline |
| Yttrium | 39 | Y | 39 | 50 | 88.906 | 4.472 | 1799 | 3609 | d- | solid | Rare Earth |
| Zirconium | 40 | Zr | 40 | 51 | 91.224 | 6.52 | 2128 | 4682 | d- | solid | metal |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Niobium | 41 | Nb | 41 | 52 | 92.906 | 8.57 | 2750 | 5017 | d- | solid | metal |
| Molybdenum | 42 | Mo | 42 | 54 | 95.95 | 10.28 | 2896 | 4912 | d- | solid | metal |
| Technetium* | 43 | Tc | 43 | 0 | [97] | 11 | 2430 | 4538 | d- | solid | metal |
| Ruthenium | 44 | Ru | 44 | 57 | 101.07 | 12.45 | 2607 | 4423 | d- | solid | metal |
| Rhodium | 45 | Rh | 45 | 58 | 102.91 | 12.41 | 2237 | 3968 | d- | solid | metal |
| Palladium | 46 | Pd | 46 | 60 | 106.42 | 12.023 | 1828.05 | 3236 | d- | solid | metal |
| Silver | 47 | Ag | 47 | 61 | 107.87 | 10.49 | 1234.93 | 2435 | d- | solid | metal |
| Cadmium | 48 | Cd | 48 | 64 | 112.41 | 8.65 | 594.22 | 1040 | d- | solid | metal |
| Indium | 49 | In | 49 | 66 | 114.82 | 7.31 | 429.75 | 2345 | p- | solid | metal |
| Tin | 50 | Sn | 50 | 69 | 118.71 | 7.265 | 505.08 | 2875 | p- | solid | metal |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Antimony | 51 | Sb | 51 | 71 | 121.76 | 6.697 | 903.78 | 1860 | p- | solid | metalloid |
| Tellurium | 52 | Te | 52 | 76 | 127.6 | 6.24 | 722.66 | 1261 | p- | solid | metalloid |
| Iodine | 53 | I | 53 | 74 | 126.9 | 4.933 | 386.85 | 457.4 | p- | solid | halogen |
| Xenon | 54 | Xe | 54 | 77 | 131.29 | 0.005894 | 161.4 | 165.03 | p- | solid | inert |
| Cesium | 55 | Cs | 55 | 78 | 132.91 | 1.93 | 301.59 | 944 | s- | solid | alkali |
| Barium | 56 | Ba | 56 | 81 | 137.33 | 3.51 | 1000 | 2170 | s- | solid | alkaline |
| Lanthanum | 57 | La | 57 | 82 | 138.91 | 6.162 | 1193 | 3737 | f- | solid | Rare Earth |
| Cerium | 58 | Ce | 58 | 82 | 140.12 | 6.77 | 1068 | 3716 | f- | solid | Rare Earth |
| Praseodymium | 59 | Pr | 59 | 82 | 140.91 | 6.77 | 1208 | 3793 | f- | solid | Rare Earth |
| Neodymium | 60 | Nd | 60 | 84 | 144.24 | 7.01 | 1297 | 3347 | f- | solid | Rare Earth |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Promethium* | 61 | Pm | 61 | 84 | [145] | 7.26 | 1315 | 3273 | f- | solid | Rare Earth |
| Samarium | 62 | Sm | 62 | 88 | 150.36 | 7.52 | 1345 | 2067 | f- | solid | Rare Earth |
| Europium | 63 | Eu | 63 | 89 | 151.96 | 5.244 | 1099 | 1802 | f- | solid | Rare Earth |
| Gadolinium | 64 | Gd | 64 | 93 | 157.25 | 7.9 | 1585 | 3546 | f- | solid | Rare Earth |
| Terbium | 65 | Tb | 65 | 94 | 158.93 | 8.23 | 1629 | 3503 | f- | solid | Rare Earth |
| Dysprosium | 66 | Dy | 66 | 97 | 162.5 | 8.54 | 1680 | 2840 | f- | solid | Rare Earth |
| Holmium | 67 | Ho | 67 | 98 | 164.93 | 8.79 | 1734 | 2993 | f- | solid | Rare Earth |
| Erbium | 68 | Er | 68 | 99 | 167.26 | 9.066 | 1802 | 3141 | f- | solid | Rare Earth |
| Thulium | 69 | Tm | 69 | 100 | 168.93 | 9.32 | 1818 | 2223 | f- | solid | Rare Earth |
| Ytterbium | 70 | Yb | 70 | 103 | 173.05 | 6.9 | 1097 | 1469 | f- | solid | Rare Earth |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Lutetium | 71 | Lu | 71 | 104 | 174.97 | 9.841 | 1925 | 3675 | d- | solid | Rare Earth |
| Hafnium | 72 | Hf | 72 | 106 | 178.49 | 13.31 | 2506 | 4876 | d- | solid | metal |
| Tantalum | 73 | Ta | 73 | 108 | 180.95 | 16.69 | 3290 | 5731 | d- | solid | metal |
| Tungsten | 74 | W | 74 | 110 | 183.84 | 19.25 | 3695 | 6203 | d- | solid | metal |
| Radium | 75 | Ra | 75 | 111 | 186.21 | 21.02 | 3459 | 5869 | d- | solid | metal |
| Osmium | 76 | Os | 76 | 114 | 190.23 | 22.59 | 3306 | 5285 | d- | solid | metal |
| Iridium | 77 | Ir | 77 | 115 | 192.22 | 22.56 | 2719 | 4701 | d- | solid | metal |
| Platinum | 78 | Pt | 78 | 117 | 195.08 | 21.45 | 2041.4 | 4098 | d- | solid | metal |
| Gold | 79 | Au | 79 | 118 | 196.97 | 19.3 | 1337.33 | 3129 | d- | solid | metal |
| Mercury | 80 | Hg | 80 | 121 | 200.59 | 13.534 | 234.43 | 629.88 | d- | liquid | metal |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Thallium | 81 | Tl | 81 | 123 | 204.38 | 11.85 | 577 | 1746 | p- | solid | metal |
| Lead | 82 | Pb | 82 | 125 | 207.2 | 11.34 | 600.61 | 2022 | p- | solid | metal |
| Bismuth | 83 | Bi | 83 | 126 | 208.98 | 9.78 | 544.7 | 1837 | p- | solid | metal |
| Polonium* | 84 | Po | 84 | 125 | [209] | 9.196 | 527 | 1235 | p- | solid | metal |
| Astatine* | 85 | At | 85 | 136 | [210] | -8.93 | 575 | 610 | p- | solid | halogen |
| Radon* | 86 | Rn | 86 | 136 | [222] | 0.00973 | 202 | 211.3 | p- | solid | inert |
| Francium* | 87 | Fr | 87 | 138 | [223] | 2.48 | 281 | 890 | s- | solid | alkali |
| Radium* | 88 | Ra | 88 | 138 | [226] | 5.5 | 973 | 2010 | s- | solid | alkaline |
| Actinium* | 89 | Ac | 89 | 142 | [227] | 10 | 1323 | 3471 | f- | solid | Actinide |
| Thorium* | 90 | Th | 90 | 140 | 232.04 | 11.7 | 2115 | 5061 | f- | solid | Actinide |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Protactinium* | 91 | Pa | 91 | 146 | 231.04 | 15.37 | 1841 | 4300 | f- | solid | Actinide |
| Uranium* | 92 | U | 92 | 144 | 238.03 | 19.1 | 1405.3 | 4404 | f- | solid | Actinide |
| Neptunium* | 93 | Np | 93 | 148 | [237] | 20.45 | 917 | 4273 | f- | solid | Actinide |
| Plutonium* | 94 | Pu | 94 | 148 | [244] | 19.85 | 912.5 | 3501 | f- | solid | Actinide |
| Americium* | 95 | Am | 95 | 151 | [243] | 12 | 1449 | 2880 | f- | solid | Actinide |
| Curium* | 96 | Cm | 96 | 150 | [247] | 13.51 | 1613 | 3383 | f- | solid | Actinide |
| Berkelium* | 97 | Bk | 97 | 153 | [247] | 14.78 | 1259 | 2900 | f- | solid | Actinide |
| Californium* | 98 | Cf | 98 | 153 | [251] | 15.1 | 1173 | -1743 | f- | solid | Actinide |
| Einsteinium* | 99 | Es | 99 | 157 | [252] | 8.84 | 1133 | -1269 | f- | solid | Actinide |
| Fermium* | 100 | Fm | 100 | 157 | [257] | -9.7 | -1125 | f- | solid | Actinide | |
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Mendelevium* | 101 | Md | 101 | 148 | [258] | -10.3 | -1100 | f- | solid | Actinide | |
| Nobelium* | 102 | No | 102 | 157 | [259] | -9.9 | -1100 | f- | solid | Actinide | |
| Lawrencium* | 103 | Lr | 103 | 157 | [266] | -14.4 | -1900 | d- | solid | Actinide | |
| ALL below ↓ are also radioactive. | |||||||||||
| Rutherfordium* | 104 | Rf | 104 | 157 | [267] | -17 | -2400 | d- | solid | ||
| Dubnium* | 105 | Db | 105 | 155 | [268] | -21.6 | d- | ||||
| Seaborgium* | 106 | Sg | 106 | 147 | [267] | (23–24) | d- | ||||
| Bohrium* | 107 | Bh | 107 | 147 | [270] | (26–27) | d- | ||||
| Hassium* | 108 | Hs | 108 | 159 | [271] | (27–29) | d- | ||||
| Meitnerium* | 109 | Mt | 109 | 161 | [278] | (27–28) | d- | ||||
| Darmstadtium* | 110 | Ds | 110 | 165 | [281] | (26–27) | d- | ||||
| Name | AN | AS | Z | N | AW | Density | Melts | Boil | Block | Phase | Type |
| Roentgenium* | 111 | Rg | 111 | [282] | (22–24) | d- | |||||
| Copernicium* | 112 | Cn | 112 | [285] | -14 | (283±11) | (340±10) | d- | |||
| Nihonium* | 113 | Nh | 113 | [286] | -16 | p- | |||||
| Flerovium* | 114 | Fl | 114 | [289] | (11.4±0.3) | (284±50) | p- | ||||
| Moscovium* | 115 | Mc | 115 | [290] | -13.5 | p- | |||||
| Livermorium* | 116 | Lv | 115 | [293] | -12.9 | p- | |||||
| Tennessine* | 117 | Ts | 117 | [294] | (7.1–7.3) | p- | |||||
| Oganassen* | 118 | Og | 118 | [294] | -7 | (325±15) | (450±10) | p- | |||
| Helium does not solidify until it is under 4 atm of pressure, then it solidifies/melts at ≈0.95K | |||||||||||
| Gallium is liquid at 303K (29.85°C; 85.73°F). You can hold in your hand and will melt, in time. | |||||||||||
| Phase is the state at ambient temperature of 294K and 1 atm. | |||||||||||
| Alkali and Alkaline tend to be highly reactive. Pure sodium, if large enough, can explode like a bomb when put into water. Even distilled. | |||||||||||
| Metalloids are actually mineral elements that also behave like a metallic element. | |||||||||||
| Halogens are gases that are highly reactive. In fact, they will react with virtually anything, even in a completely anoxic atmosphere. | |||||||||||
| Inert gases are just that. Inert. They do not react with anything. | |||||||||||
| I am not sure what the negative density for Astatine is. I just downloaded the table in csv, then loaded into sprdsht. | |||||||||||
| HA! Anoxic was spelt correct. I have seen it spelt “ænoxic”. Sprdsht flagged with red squiggly. | |||||||||||
| ALL actinides are radioactive. | |||||||||||
| Naturally occurring radioactive elements. | |||||||||||
| Artificially generated radioactive elements. | |||||||||||
— The Unknown Atheist
Copyright © 2024 by RMFR. Licensed under CC-BY-NC-SA 4.0 International. All Other Rights Reserved